-
PDF
- Split View
-
Views
-
Cite
Cite
Jane E. Ferrie, Mika Kivimäki, Tasnime N. Akbaraly, Archana Singh-Manoux, Michelle A. Miller, David Gimeno, Meena Kumari, George Davey Smith, Martin J. Shipley, Associations Between Change in Sleep Duration and Inflammation: Findings on C-reactive Protein and Interleukin 6 in the Whitehall II Study, American Journal of Epidemiology, Volume 178, Issue 6, 15 September 2013, Pages 956–961, https://doi.org/10.1093/aje/kwt072
Close - Share Icon Share
Abstract
Cross-sectional evidence suggests associations between sleep duration and levels of the inflammatory markers, C-reactive protein and interleukin-6. This longitudinal study uses data from the London-based Whitehall II study to examine whether changes in sleep duration are associated with average levels of inflammation from 2 measures 5 years apart. Sleep duration (≤5, 6, 7, 8, ≥9 hours on an average week night) was assessed in 5,003 middle-aged women and men in 1991/1994 and 1997/1999. Fasting levels of C-reactive protein and interleukin-6 were measured in 1997/1999 and 2002/2004. Cross-sectional analyses indicated that shorter sleep is associated with higher levels of inflammatory markers. Longitudinal analyses showed that each hour per night decrease in sleep duration between 1991/1994 and 1997/1999 was associated with higher levels of C-reactive protein (8.1%) and interleukin-6 (4.5%) averaged across measures in 1997/1999 and 2002/2004. Adjustment for longstanding illness and major cardiometabolic risk factors indicated that disease processes may partially underlie these associations. An increase in sleep duration was not associated with average levels of inflammatory markers. These results suggest that both short sleep and reductions in sleep are associated with average levels of inflammation over a 5-year period.
Poor sleep is associated with adverse health outcomes, including type 2 diabetes (1), cardiovascular disease (2), and premature death (3–5). Inflammation plays an important role in the development of many diseases (6). For example, C-reactive protein, a nonspecific marker of acute-phase inflammatory response, and interleukin-6, which regulates C-reactive protein synthesis (7), have been shown to predict cardiovascular morbidity, and Mendelian randomization analyses suggest that associations with interleukin-6 may be causal (8–11).
Poor sleep can result from disease processes involving inflammation (12). However, it has recently been suggested that sleep curtailment might also induce inflammatory responses outside the disease process. Thus, associations between poor sleep and disease may be partially mediated by long-term inflammatory mechanisms, either directly or indirectly via effects on risk factors like hypertension (13–16). To date, however, studies of sleep and levels of inflammation assessed over several years using repeat measurements of inflammatory markers are largely lacking.
In this paper, we examine whether changes in sleep duration over 5 years are associated with average levels of C-reactive protein and interleukin-6 assessed twice over the subsequent 5 years in a large, prospective study of middle-aged women and men.
MATERIALS AND METHODS
Study population
The target population for the Whitehall II study was all London-based office staff aged 35–55 years in 20 civil service departments in 1985. Of these, 10,308 enrolled, a response rate of 73% (17). Data collection at enrollment and in 1991/1994, 1997/1999, and 2002/2004 involved a clinical examination and self-administered questionnaire. The current study examines associations of sleep duration in 1997/1999 and changes in sleep duration between 1991/1994 and 1997/1999 with subsequent levels of C-reactive protein and interleukin-6 averaged across measures in 1997/1999 and 2002/2004.
Assessment of exposure, outcome, and covariates
Sleep duration was measured by using the question, “How many hours of sleep do you have on an average week night?” Response categories were ≤5, 6, 7, 8, and ≥9. Changes in sleep duration from 1991/1994 to 1997/1999 were categorized by whether participants slept more, the same, or less hours. Outcomes were levels of C-reactive protein and interleukin-6 measured in fasting serum as described previously (18), averaged over measurements in 1997/1999 and 2002/2004 as an estimate of the chronic inflammation that has been shown to be associated with the development of cardiometabolic disease and depression (19, 20). Values lower than the detection limit, 0.154 mg/L (C-reactive protein) and 0.08 pg/mL (interleukin-6), were assigned values equal to half the detection limit. Covariates (from 1997/1999) included age, sex, occupational position, systolic blood pressure, body mass index, total cholesterol concentration, and diabetes (17).
Statistical analysis
Distributions of average C-reactive protein and interleukin-6 were normalized by logarithmic transformation. Multiple regression models with C-reactive protein and interleukin-6 as the dependent variables and sleep duration categories as the independent variables were fitted to estimate the following: the means adjusted for age, sex, and occupational position; tests of heterogeneity across the sleep duration categories; and tests of difference in C-reactive protein or interleukin-6 compared with the sleep duration category of ≤5 hours.
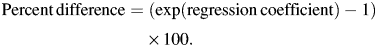
Models were serially adjusted by the covariates to examine effects on the associations. As no interaction between sex and sleep duration in relation to C-reactive protein (P = 0.84) or interleukin-6 (P = 0.44) was detected, women and men participants were combined. All analyses were conducted by using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Compared with the 3,812 participants enrolled but not contributing to the analyses, the 5,003 included were more likely to be men (71.8% vs. 64.7%), younger (49.3 vs. 50.4 years), and short (≤5 hours/night) or long (≥9 hours/night) sleepers, as well as less likely to have a low occupational position (11.8% vs. 24.4%), At baseline in 1991/1994, sleep duration was 6 or 7 hours/night for 68.3% of participants; short sleep was reported by 3.6% and long sleep by 2.9%. Sleep duration between 1991/1994 and 1997/1999 remained unchanged for 48.1%, while change >1 hour was observed in 9.2%.
Sleep duration
Table 1 shows the distribution of average levels of C-reactive protein and interleukin-6 in 1997/1999 and 2002/2004 by sleep duration in 1997/1999, adjusted for age, sex, and occupational position. Longer sleep durations were associated with lower C-reactive protein and interleukin-6. Participants sleeping ≥9 hours departed from this trend but were too few for meaningful analysis.
Association Between Sleep Duration, Assessed in 1997/1999, and Average C-reactive Protein and Interleukin-6, Assessed in 1997/1999 and 2002/2004 Using Data From the Whitehall II Study
| Sleep Duration, hours . | C-reactive Protein (mg/L) . | ||
|---|---|---|---|
| No. . | Meana . | 95% CI . | |
| Total | 4,972 | ||
| ≤5 | 365 | 1.47 | 1.32, 1.64 |
| 6 | 1,638 | 1.30* | 1.23, 1.36 |
| 7 | 2,149 | 1.28* | 1.22, 1.34 |
| 8 | 747 | 1.20** | 1.11, 1.29 |
| ≥9 | 73 | 1.31 | 1.03, 1.66 |
| Pheterogeneity | 0.05 | ||
| . | Interleukin-6 (µg/L) . | ||
| . | No. . | Meana . | 95% CI . |
| Total | 4,689 | ||
| ≤5 | 339 | 1.85 | 1.75, 1.96 |
| 6 | 1,558 | 1.75 | 1.71, 1.80 |
| 7 | 2,027 | 1.70** | 1.67, 1.74 |
| 8 | 698 | 1.69** | 1.62, 1.75 |
| ≥9 | 67 | 1.74 | 1.54, 1.97 |
| Pheterogeneity | 0.04 | ||
| Sleep Duration, hours . | C-reactive Protein (mg/L) . | ||
|---|---|---|---|
| No. . | Meana . | 95% CI . | |
| Total | 4,972 | ||
| ≤5 | 365 | 1.47 | 1.32, 1.64 |
| 6 | 1,638 | 1.30* | 1.23, 1.36 |
| 7 | 2,149 | 1.28* | 1.22, 1.34 |
| 8 | 747 | 1.20** | 1.11, 1.29 |
| ≥9 | 73 | 1.31 | 1.03, 1.66 |
| Pheterogeneity | 0.05 | ||
| . | Interleukin-6 (µg/L) . | ||
| . | No. . | Meana . | 95% CI . |
| Total | 4,689 | ||
| ≤5 | 339 | 1.85 | 1.75, 1.96 |
| 6 | 1,558 | 1.75 | 1.71, 1.80 |
| 7 | 2,027 | 1.70** | 1.67, 1.74 |
| 8 | 698 | 1.69** | 1.62, 1.75 |
| ≥9 | 67 | 1.74 | 1.54, 1.97 |
| Pheterogeneity | 0.04 | ||
Abbreviation: CI, confidence interval.
* P < 0.05; **P < 0.01 (difference relative to ≤5 hours, arbitrary reference category).
a Values are geometric means adjusted for age, sex, and occupational position.
Association Between Sleep Duration, Assessed in 1997/1999, and Average C-reactive Protein and Interleukin-6, Assessed in 1997/1999 and 2002/2004 Using Data From the Whitehall II Study
| Sleep Duration, hours . | C-reactive Protein (mg/L) . | ||
|---|---|---|---|
| No. . | Meana . | 95% CI . | |
| Total | 4,972 | ||
| ≤5 | 365 | 1.47 | 1.32, 1.64 |
| 6 | 1,638 | 1.30* | 1.23, 1.36 |
| 7 | 2,149 | 1.28* | 1.22, 1.34 |
| 8 | 747 | 1.20** | 1.11, 1.29 |
| ≥9 | 73 | 1.31 | 1.03, 1.66 |
| Pheterogeneity | 0.05 | ||
| . | Interleukin-6 (µg/L) . | ||
| . | No. . | Meana . | 95% CI . |
| Total | 4,689 | ||
| ≤5 | 339 | 1.85 | 1.75, 1.96 |
| 6 | 1,558 | 1.75 | 1.71, 1.80 |
| 7 | 2,027 | 1.70** | 1.67, 1.74 |
| 8 | 698 | 1.69** | 1.62, 1.75 |
| ≥9 | 67 | 1.74 | 1.54, 1.97 |
| Pheterogeneity | 0.04 | ||
| Sleep Duration, hours . | C-reactive Protein (mg/L) . | ||
|---|---|---|---|
| No. . | Meana . | 95% CI . | |
| Total | 4,972 | ||
| ≤5 | 365 | 1.47 | 1.32, 1.64 |
| 6 | 1,638 | 1.30* | 1.23, 1.36 |
| 7 | 2,149 | 1.28* | 1.22, 1.34 |
| 8 | 747 | 1.20** | 1.11, 1.29 |
| ≥9 | 73 | 1.31 | 1.03, 1.66 |
| Pheterogeneity | 0.05 | ||
| . | Interleukin-6 (µg/L) . | ||
| . | No. . | Meana . | 95% CI . |
| Total | 4,689 | ||
| ≤5 | 339 | 1.85 | 1.75, 1.96 |
| 6 | 1,558 | 1.75 | 1.71, 1.80 |
| 7 | 2,027 | 1.70** | 1.67, 1.74 |
| 8 | 698 | 1.69** | 1.62, 1.75 |
| ≥9 | 67 | 1.74 | 1.54, 1.97 |
| Pheterogeneity | 0.04 | ||
Abbreviation: CI, confidence interval.
* P < 0.05; **P < 0.01 (difference relative to ≤5 hours, arbitrary reference category).
a Values are geometric means adjusted for age, sex, and occupational position.
Change in sleep duration
Table 2 shows the percent difference in average C-reactive protein and interleukin-6 levels in 1997/1999 and 2002/2004 associated with change in sleep duration, separately for increased and decreased sleep. Each 1-hour decrease was associated with an 8.1% higher level of C-reactive protein and 4.5% higher level of interleukin-6 adjusted for age and sex. Adjustment for occupational position slightly attenuated these associations. Further adjustment for systolic blood pressure, body mass index, total cholesterol, and diabetes resulted in a total attenuation of 48% for C-reactive protein and 40% for interleukin-6. Increases in sleep duration were not associated with either inflammatory marker.
Percentage Difference in Average C-reactive Protein and Interleukin-6 (Assessed in 1997/1999 and 2002/2004) Per 1-Hour Change in Duration of Sleep Between 1991/1994 and 1997/1999 Using Data From the Whitehall II Study
| . | Decreased Sleep . | Increased Sleep . | ||||
|---|---|---|---|---|---|---|
| . | Difference Per Hour, % . | 95% CI . | P Value . | Difference Per Hour, % . | 95% CI . | P Value . |
| Model 1a | ||||||
| C-reactive proteinb | 8.1 | 2.6, 13.9 | 0.004 | −3.6 | −11.7, 5.3 | 0.42 |
| Interleukin-6c | 4.5 | 1.7, 7.4 | 0.001 | −1.4 | −5.7, 3.1 | 0.54 |
| Model 2d | ||||||
| C-reactive protein | 6.7 | 1.3, 12.5 | 0.01 | −3.5 | −11.5, 5.3 | 0.43 |
| Interleukin-6 | 3.6 | 0.8, 6.4 | 0.01 | −1.4 | −5.7, 3.1 | 0.53 |
| Model 3e | ||||||
| C-reactive protein | 4.2 | −0.9, 9.4 | 0.11 | −2.4 | −10.1, 5.9 | 0.56 |
| Interleukin-6 | 2.7 | 0.1, 5.3 | 0.04 | −0.7 | −4.8, 3.6 | 0.56 |
| . | Decreased Sleep . | Increased Sleep . | ||||
|---|---|---|---|---|---|---|
| . | Difference Per Hour, % . | 95% CI . | P Value . | Difference Per Hour, % . | 95% CI . | P Value . |
| Model 1a | ||||||
| C-reactive proteinb | 8.1 | 2.6, 13.9 | 0.004 | −3.6 | −11.7, 5.3 | 0.42 |
| Interleukin-6c | 4.5 | 1.7, 7.4 | 0.001 | −1.4 | −5.7, 3.1 | 0.54 |
| Model 2d | ||||||
| C-reactive protein | 6.7 | 1.3, 12.5 | 0.01 | −3.5 | −11.5, 5.3 | 0.43 |
| Interleukin-6 | 3.6 | 0.8, 6.4 | 0.01 | −1.4 | −5.7, 3.1 | 0.53 |
| Model 3e | ||||||
| C-reactive protein | 4.2 | −0.9, 9.4 | 0.11 | −2.4 | −10.1, 5.9 | 0.56 |
| Interleukin-6 | 2.7 | 0.1, 5.3 | 0.04 | −0.7 | −4.8, 3.6 | 0.56 |
Abbreviation: CI, confidence interval.
a Model 1 is adjusted for age and sex.
b The numbers of participants with decreased hours, same hours, and increased hours of sleep between 1991/1994 and 1997/1999 are 1,757, 2,286, and 592, respectively.
c The numbers of participants with decreased hours, same hours, and increased hours of sleep between 1991/1994 and 1997/1999 are 1,657, 2,162, and 561, respectively.
d Model 2 is adjusted for age, sex, and occupational position in 1997/1999.
e Model 3 is adjusted for age, sex, occupational position, systolic blood pressure, body mass index, cholesterol concentration, and diabetes in 1997/1999.
Percentage Difference in Average C-reactive Protein and Interleukin-6 (Assessed in 1997/1999 and 2002/2004) Per 1-Hour Change in Duration of Sleep Between 1991/1994 and 1997/1999 Using Data From the Whitehall II Study
| . | Decreased Sleep . | Increased Sleep . | ||||
|---|---|---|---|---|---|---|
| . | Difference Per Hour, % . | 95% CI . | P Value . | Difference Per Hour, % . | 95% CI . | P Value . |
| Model 1a | ||||||
| C-reactive proteinb | 8.1 | 2.6, 13.9 | 0.004 | −3.6 | −11.7, 5.3 | 0.42 |
| Interleukin-6c | 4.5 | 1.7, 7.4 | 0.001 | −1.4 | −5.7, 3.1 | 0.54 |
| Model 2d | ||||||
| C-reactive protein | 6.7 | 1.3, 12.5 | 0.01 | −3.5 | −11.5, 5.3 | 0.43 |
| Interleukin-6 | 3.6 | 0.8, 6.4 | 0.01 | −1.4 | −5.7, 3.1 | 0.53 |
| Model 3e | ||||||
| C-reactive protein | 4.2 | −0.9, 9.4 | 0.11 | −2.4 | −10.1, 5.9 | 0.56 |
| Interleukin-6 | 2.7 | 0.1, 5.3 | 0.04 | −0.7 | −4.8, 3.6 | 0.56 |
| . | Decreased Sleep . | Increased Sleep . | ||||
|---|---|---|---|---|---|---|
| . | Difference Per Hour, % . | 95% CI . | P Value . | Difference Per Hour, % . | 95% CI . | P Value . |
| Model 1a | ||||||
| C-reactive proteinb | 8.1 | 2.6, 13.9 | 0.004 | −3.6 | −11.7, 5.3 | 0.42 |
| Interleukin-6c | 4.5 | 1.7, 7.4 | 0.001 | −1.4 | −5.7, 3.1 | 0.54 |
| Model 2d | ||||||
| C-reactive protein | 6.7 | 1.3, 12.5 | 0.01 | −3.5 | −11.5, 5.3 | 0.43 |
| Interleukin-6 | 3.6 | 0.8, 6.4 | 0.01 | −1.4 | −5.7, 3.1 | 0.53 |
| Model 3e | ||||||
| C-reactive protein | 4.2 | −0.9, 9.4 | 0.11 | −2.4 | −10.1, 5.9 | 0.56 |
| Interleukin-6 | 2.7 | 0.1, 5.3 | 0.04 | −0.7 | −4.8, 3.6 | 0.56 |
Abbreviation: CI, confidence interval.
a Model 1 is adjusted for age and sex.
b The numbers of participants with decreased hours, same hours, and increased hours of sleep between 1991/1994 and 1997/1999 are 1,757, 2,286, and 592, respectively.
c The numbers of participants with decreased hours, same hours, and increased hours of sleep between 1991/1994 and 1997/1999 are 1,657, 2,162, and 561, respectively.
d Model 2 is adjusted for age, sex, and occupational position in 1997/1999.
e Model 3 is adjusted for age, sex, occupational position, systolic blood pressure, body mass index, cholesterol concentration, and diabetes in 1997/1999.
Sensitivity analyses
Removal of C-reactive protein values over 10 mg/L, a level considered consistent with acute inflammation (7), had little effect on our findings. Each hour decrease in sleep/night was associated with a 7.8% (95% confidence interval (CI): 2.8, 13.1) and a 6.3% (95% CI: 1.4, 11.5) higher level of C-reactive protein in analyses adjusted for age and sex and for age, sex, and occupational position.
To test whether observed associations between decreased sleep and higher levels of C-reactive protein and interleukin-6 were due to prevalent disease, we ran analyses adjusted for longstanding illness (1991/1994): The percent increase in C-reactive protein/hour of sleep lost was 5.8% (95% CI: 0.5, 11.5) (P = 0.03) and in interleukin-6 was 3.3% (95% CI: 0.6, 6.1) (P = 0.02). After additional adjustment for systolic blood pressure, body mass index, total cholesterol, and diabetes, the corresponding values were 3.3% (95% CI: −1.6, 8.5) (P = 0.19) and 2.5% (95% CI: −0.1, 5.2) (P = 0.06), respectively. Excluding participants (C-reactive protein, n = 72; interleukin-6, n = 62) with incident cancer in 1991/1994–1997/1999 had little effect on these estimates.
Additional sensitivity analyses showed no association between decreased sleep duration between 1991/1994 and 1997/1999 and changes in C-reactive protein and interleukin-6 over the follow-up for inflammatory markers (data not shown). However, there was an association (P = 0.02) between increased sleep and a decrease in C-reactive protein over follow-up, possibly indicating a beneficial effect of increased sleep on C-reactive protein in 2002/2004 compared with 1997/1999.
DISCUSSION
Our findings suggest that shorter sleep and decreases in sleep duration are associated with higher levels of C-reactive protein and interleukin-6, assessed twice over the subsequent 5-year period, in analyses adjusted for age, sex, and occupational position. Further adjustment for longstanding illness and major cardiometabolic risk factors attenuated these associations, although evidence suggesting that a decrease in sleep is associated with a higher level of interleukin-6 remained.
Previous cross-sectional investigations of sleep duration and inflammatory markers have produced mixed findings. Analysis of 1991/1994 data from the Whitehall II study showed inverse associations among sleep duration, interleukin-6, and C-reactive protein in women but not in men (18). Other cross-sectional studies in the sexes combined found no association between sleep duration and C-reactive protein (21), as well as 8% and 7% higher levels of C-reactive protein and interleukin-6 for every additional hour of sleep/night (22). Studies in women suggest an association between shorter sleep and higher C-reactive protein in middle-aged African Americans, but not in other ethnic groups (23), and no association between sleep duration and C-reactive protein or interleukin-6 in young women (24). The only previous study with a longitudinal component examined inflammatory markers as the exposure and suggested that higher levels of interleukin-6 at baseline and an increase in interleukin-6 over a 6-year period were associated with an increase in long sleep (>8 hours) in older women and men (25).
None of these studies used average measures of inflammatory markers. Recently, the importance of chronic inflammation in cardiometabolic disease etiology and depression has been recognized (19, 26, 27). An average of values over 2 time points provides a better marker of chronic inflammation than a single measurement and reduces the possibility of spurious findings due to acute infection, a possibility further reduced by removing C-reactive protein values over 10 mg/L (7). However, as differences between 2 measurements may actually represent real change, we ran a sensitivity analysis excluding those whose change in C-reactive protein or interleukin-6 was greater than 1 standard deviation at the beginning of the 5-year follow-up. This excluded 18% of participants for C-reactive protein and 24% for interleukin-6. The association between decreased sleep duration and C-reactive protein was little affected, while that with interleukin-6 was strengthened at 4.4% (95% CI: 1.5, 7.3) (P = 0.003).
In our analyses adjusted for longstanding illness and major cardiometabolic risk factors, associations between decreased sleep and C-reactive protein and interleukin-6 were reduced, indicating that poor sleep may result in part from disease processes involving inflammation. However, these analyses also suggest a role for interleukin-6 outside the disease process. Such an effect is biologically plausible, because experiments suggest that prolonged sleep deprivation in rats is associated with an evolving proinflammatory state (28), and studies in humans show that total sleep deprivation is associated specifically with increasing circulating levels of interleukin-6 (29). More common forms of sleep loss, such as reductions of 25%–50% across consecutive nights, appear to induce an increase in interleukin-6 and C-reactive protein levels (30–32), and increased levels of interleukin-6 are found in patients with primary insomnia (33, 34). Morning levels of nuclear-factor kappa beta (a protein complex linked to inflammatory response) activation, are higher after a night of sleep loss, potentially identifying nuclear-factor kappa beta transcription pathways as the molecular mechanism by which sleep influences the production of interleukin-6 and other inflammatory cytokines (35). Although relative increases in inflammatory markers in experimental studies are tiny, small basal shifts are consistent with levels of elevation seen in studies of cardiovascular risk (19). As little has been known about the effects of more persistent sleep deprivation on immune function, our finding that a decrease in sleep duration is associated with chronic inflammation at a population level is important.
The present study has at least 3 limitations. First, our sleep measure was self-reported and did not include sleep quality. There is evidence from experimental studies that sleep disturbances may also be associated with inflammation (19). However, in this paper we chose to focus on duration as no previous study has examined associations between changes in this important aspect of the circadian cycle and inflammatory markers at a population level. Large-scale studies of more objective sleep duration measures remain costly, and small-scale investigations have shown moderately good correlations between subjective estimates and sleep diaries, actigraphy, or polysomnography (36). Self-reported sleep duration is also strongly associated with objectively ascertained health outcomes (3, 4), and assessments in the primary health-care setting rely on self-reports from patients. Nevertheless, further studies are needed to examine change in sleep quality and inflammatory markers in population studies, as well as the effect of changes in combined measures of duration and quality, as these will come closer to a measure of sleep efficacy. Ultimately, population studies using polysomnography, once it has been developed to that point of unobtrusiveness at which long-term use at home is possible, will provide more definitive answers. Second, the present findings from an occupational cohort of middle-aged, white-collar civil servants may not be generalizable to blue-collar and private sector workers. Third, stressful life events, which may be associated with sleep duration and immune function, were not measured after 1989/1990 and so could not be included in the analyses.
The main strengths of the study were a longitudinal design facilitating examination of changes in sleep duration, a large population including both sexes, and levels of inflammatory markers averaged over 2 measurements to assess chronic inflammation.
In conclusion, chronic low-grade inflammation is becoming firmly established as a risk factor for cardiometabolic disease and depression. This study shows that short sleep and, more importantly, reductions in sleep duration are associated with average levels of inflammation.
ACKNOWLEDGMENTS
Author affiliations: School of Social and Community Medicine, University of Bristol, Bristol, United Kingdom (Jane E. Ferrie, George Davey Smith); Department of Epidemiology and Public Health, University College London, London, United Kingdom (Jane E. Ferrie, Tasnime N. Akbaraly, Archana Singh-Manoux, Mika Kivimäki, Meena Kumari, Martin J. Shipley); Institut National de la Santé et de la Recherche Médicale, U1061, Montpellier, France (Tasnime N. Akbaraly); University Montpellier I, Montpellier, France (Tasnime N. Akbaraly); Centre for Research in Epidemiology and Population Health, Institut National de la Santé et de la Recherche Médicale, U1018, Villjuif Cedex, France (Archana Singh-Manoux); Centre de Gérontologie, Hôpital Ste Périne, Paris, France (Archana Singh-Manoux); Warwick Medical School, University of Warwick, Coventry, United Kingdom (Michelle A. Miller); Division of Epidemiology, Human Genetics, and Environmental Sciences, Southwest Center for Occupational and Environmental Health, The University of Texas School of Public Health, San Antonio, Texas (David Gimeno); and Medical Research Council Centre for Causal Analyses in Translational Epidemiology, University of Bristol, Bristol, United Kingdom (George Davey Smith).
The Whitehall II study has been supported by grants from the Medical Research Council; British Heart Foundation; National Heart, Lung, and Blood Institute (R01HL36310), National Institute on Aging (R01AG13196 and R01AG34454), and the Agency for Health Care Policy Research (HS06516), US National Institutes of Health; the Dunhill Medical Trust (R247/0512); and the BUPA (British United Provident Association) Foundation, United Kingdom. M. Ki. is supported by the Medical Research Council (K013351), Academy of Finland, and an Economic and Social Research Council professorship. M. J. S. is supported by the British Heart Foundation. T. N. A. was supported by the National Heart, Lung, and Blood Institute (R01HL36310). M. Ku. is partly supported by the Economic and Social Research Council (RES-596-28-0001).
This work was performed at the Department of Epidemiology and Public Health, University College London Medical School.
Conflict of interest: none declared.
REFERENCES
Author notes
Abbreviation: CI, confidence interval.




